Accenture creates a regulatory document authoring solution using AWS generative AI services
AWS Machine Learning
FEBRUARY 6, 2024
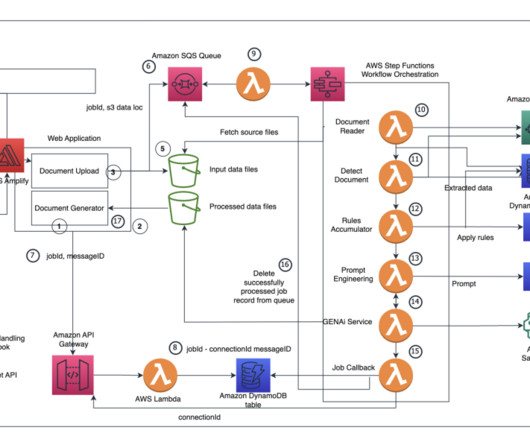
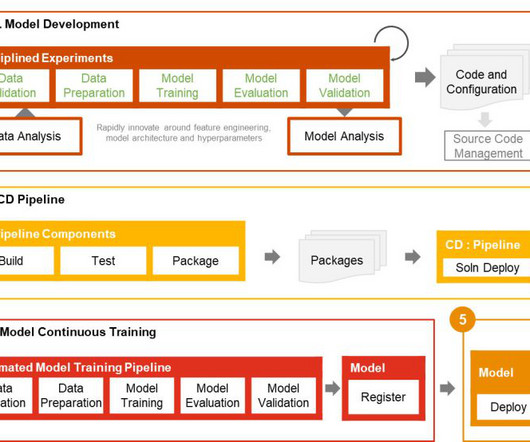
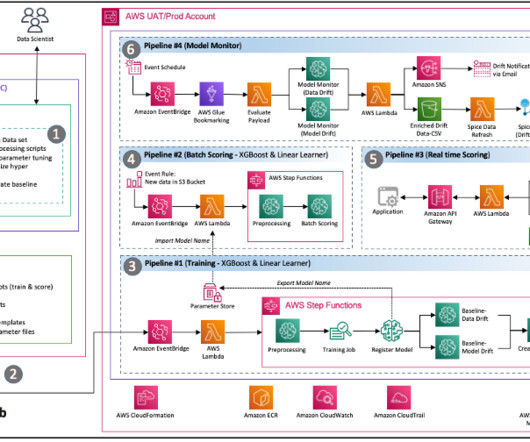
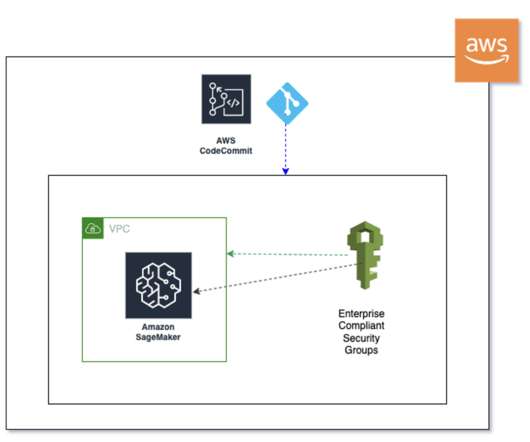
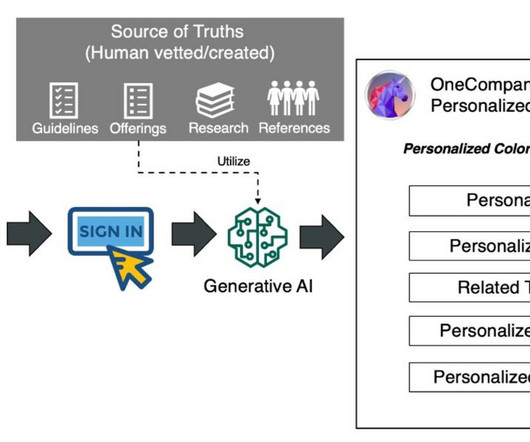
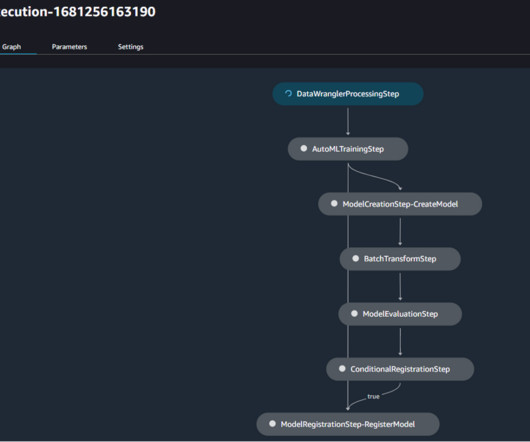
Companies face complex regulations and extensive approval requirements from governing bodies like the US Food and Drug Administration (FDA). Users then review and edit the documents, where necessary, and submit the same to the central governing bodies. This post is co-written with Ilan Geller, Shuyu Yang and Richa Gupta from Accenture.









Let's personalize your content